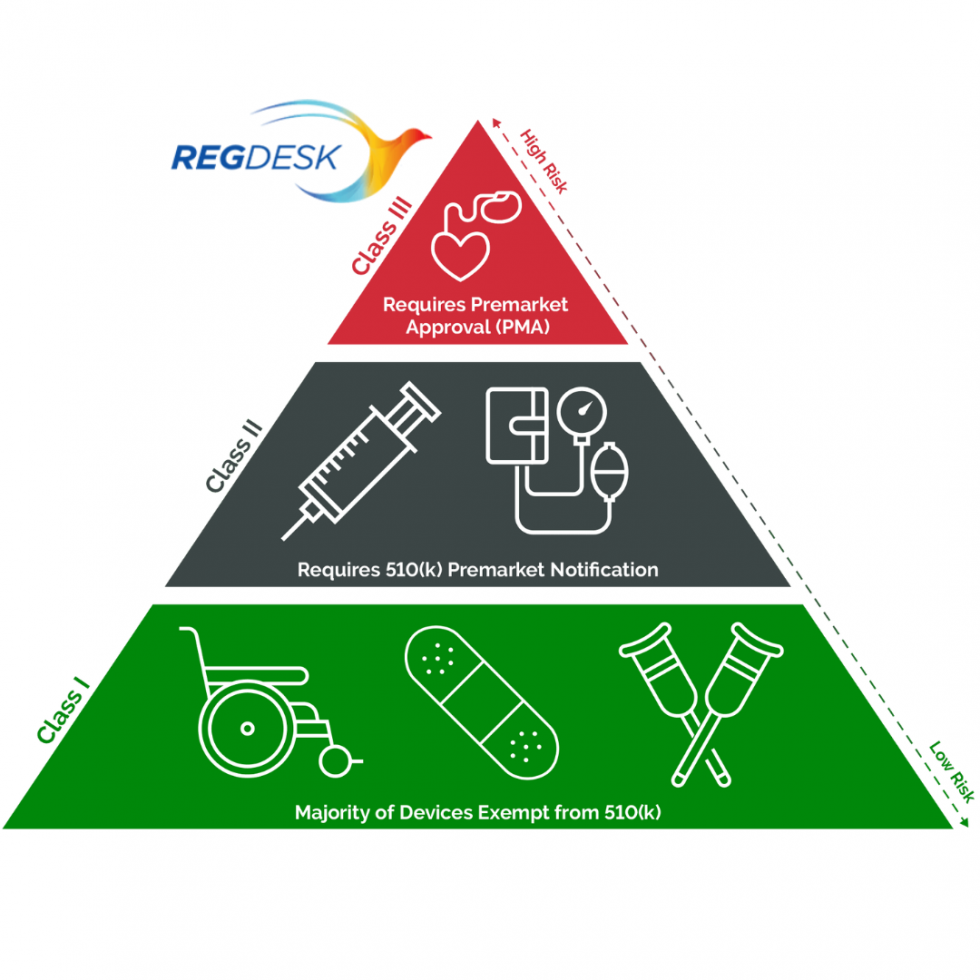
Do Medical Devices Need Fda Approval . The following information is available: Before a medical device can be legally sold in the u.s., the person. welcome to fda's information about medical device approvals. — learn how the fda regulates medical devices in the u.s. in the u.s., fda regulates the sale of medical device products. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. Find out the differences between. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). Based on their potential risks and review processes. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. How are medical devices regulated in the usa?
from www.regdesk.co
similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). The following information is available: Find out the differences between. in the u.s., fda regulates the sale of medical device products. welcome to fda's information about medical device approvals. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. — learn how the fda regulates medical devices in the u.s. Before a medical device can be legally sold in the u.s., the person. How are medical devices regulated in the usa?
Do All Medical Devices Need FDA Approval? RegDesk
Do Medical Devices Need Fda Approval similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. Based on their potential risks and review processes. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. welcome to fda's information about medical device approvals. The following information is available: How are medical devices regulated in the usa? in the u.s., fda regulates the sale of medical device products. — learn how the fda regulates medical devices in the u.s. Before a medical device can be legally sold in the u.s., the person. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. Find out the differences between.
From www.pharmacompass.com
FDA & EMA’s New Drug Approvals (Mid2021 Recap) Radio Data Compilation Do Medical Devices Need Fda Approval similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. Based on their potential risks and review processes. — learn how the fda regulates medical devices in the u.s. Find out the differences between. The following information is available: . Do Medical Devices Need Fda Approval.
From www.registrarcorp.com
How to Get FDA Approval Registrar Do Medical Devices Need Fda Approval Find out the differences between. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). Before a medical device can be legally sold in the u.s., the person. welcome to fda's information about medical device approvals. The following information is available: in the u.s., fda regulates the sale of. Do Medical Devices Need Fda Approval.
From www.medicalpriceonline.com
FDA Approval Process for Medical Devices Medical Price Online Do Medical Devices Need Fda Approval welcome to fda's information about medical device approvals. How are medical devices regulated in the usa? Before a medical device can be legally sold in the u.s., the person. in the u.s., fda regulates the sale of medical device products. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration. Do Medical Devices Need Fda Approval.
From www.pennmedicine.org
FDA Approvals In Cancer Care Penn Medicine Do Medical Devices Need Fda Approval The following information is available: — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. in the u.s., fda regulates the sale of medical device products. Find out the differences between. Based on their potential risks and review processes. similar to drugs, medical. Do Medical Devices Need Fda Approval.
From credevo.com
Medical Device Market Approval Process in the United States Credevo Do Medical Devices Need Fda Approval Find out the differences between. welcome to fda's information about medical device approvals. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). Based on. Do Medical Devices Need Fda Approval.
From qbdgroup.com
The Advent of Artificial Intelligence & Machine Learning in Medical Devices Do Medical Devices Need Fda Approval welcome to fda's information about medical device approvals. in the u.s., fda regulates the sale of medical device products. The following information is available: Find out the differences between. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for.. Do Medical Devices Need Fda Approval.
From www.mokomedtech.com
How to Get FDA Approval for Medical Devices MokoMedtech Professional Do Medical Devices Need Fda Approval similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. — learn how the fda regulates medical devices in the u.s. Before a medical device can be legally sold in the u.s., the person. — the devices do need. Do Medical Devices Need Fda Approval.
From www.linkedin.com
FDA's AI Medical Device Approvals Do Medical Devices Need Fda Approval similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. How are medical devices regulated in the usa? The following information is available: — the devices do need fda “clearance” before they can be marketed and sold, but rather than. Do Medical Devices Need Fda Approval.
From www.cognidox.com
The FDA submission process 510K vs PMA. What’s the difference? Do Medical Devices Need Fda Approval How are medical devices regulated in the usa? — learn how the fda regulates medical devices in the u.s. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. similar to drugs, medical devices in the united states go through a review process. Do Medical Devices Need Fda Approval.
From angelanjohnson.com
Medical Devices Angela N Johnson Do Medical Devices Need Fda Approval — learn how the fda regulates medical devices in the u.s. Before a medical device can be legally sold in the u.s., the person. How are medical devices regulated in the usa? Find out the differences between. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration. Do Medical Devices Need Fda Approval.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Do Medical Devices Need Fda Approval in the u.s., fda regulates the sale of medical device products. The following information is available: welcome to fda's information about medical device approvals. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). — learn how the fda regulates medical devices in the u.s. Before a medical. Do Medical Devices Need Fda Approval.
From www.pinterest.com
Fdahelp provides to you FDA approval service in USA. https//www Do Medical Devices Need Fda Approval in the u.s., fda regulates the sale of medical device products. How are medical devices regulated in the usa? — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. similar to drugs, medical devices in the united states go through a review process. Do Medical Devices Need Fda Approval.
From www.parasoft.com
Prepare your medical device software for the new FDA cybersecurity guidance Do Medical Devices Need Fda Approval — learn how the fda regulates medical devices in the u.s. Find out the differences between. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. in the u.s., fda regulates the sale of medical device products. Before a. Do Medical Devices Need Fda Approval.
From aaos.org
An Overview of the FDA Approval Process for Devices Do Medical Devices Need Fda Approval Find out the differences between. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). Based on their potential risks and review. Do Medical Devices Need Fda Approval.
From webinars.sw.siemens.com
Navigating the FDA Approval Process for Your Software Based Medical De Do Medical Devices Need Fda Approval Find out the differences between. Based on their potential risks and review processes. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical trial, the. similar to drugs, medical devices in the united states go through a review process by the us food and drug administration. Do Medical Devices Need Fda Approval.
From pathwaynpi.com
Do Medical Devices Need FDA Approval? Pathway NPI Pathway New Do Medical Devices Need Fda Approval How are medical devices regulated in the usa? similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. — the devices do need fda “clearance” before they can be marketed and sold, but rather than submit their products for clinical. Do Medical Devices Need Fda Approval.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Do Medical Devices Need Fda Approval How are medical devices regulated in the usa? In the united states, all medical devices’ safety and effectiveness are regulated by the food and drug administration (fda). welcome to fda's information about medical device approvals. — learn how the fda regulates medical devices in the u.s. Before a medical device can be legally sold in the u.s., the. Do Medical Devices Need Fda Approval.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Do Medical Devices Need Fda Approval similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for. The following information is available: Before a medical device can be legally sold in the u.s., the person. Based on their potential risks and review processes. Find out the differences between.. Do Medical Devices Need Fda Approval.